Cleanroom Classifications and Standards
Introduction
Cleanroom classifications and standards are essential for industries such as pharmaceuticals, microelectronics, biotechnology, and aerospace, controlling contamination is paramount. These classifications define the allowable number and size of particles in a cleanroom and are regulated by international and national standards. Like ISO 14644-1, Federal Standard 209E, and more. Understand their significance for industries is important for designing & maintaining these cleanroom environments effectively.
We will compare the major world cleanroom classifications, like ISO 14644-1 and Federal Standard 209E, and explore best practices to ensure compliance.
What Are Cleanroom Classifications?
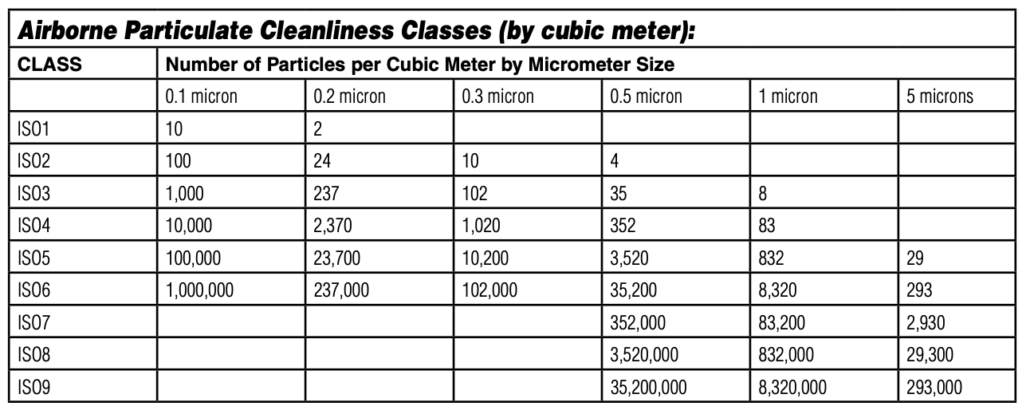
Cleanroom classifications are defined based on the number of airborne particles per cubic meter or cubic foot at specific particle sizes. These classifications dictate how “clean” the environment is and ensure it meets the stringent requirements of various industries. The most widely recognized cleanroom classification standards are:
ISO 14644-1: It is the Global Standard,The international standard that classifies cleanrooms based on particle size and concentration.
The ISO 14644-1 standard is recognized globally and widely used in industries where cleanroom environments are necessary. It specifies the cleanliness of air based on the maximum allowable particle concentration for different particle sizes.
Federal Standard 209E: The now-obsolete U.S. standard, still used by some companies, that classifies cleanrooms based on particle concentration (≥0.5 µm per cubic foot).
Federal Standard 209E vs ISO 14644-1
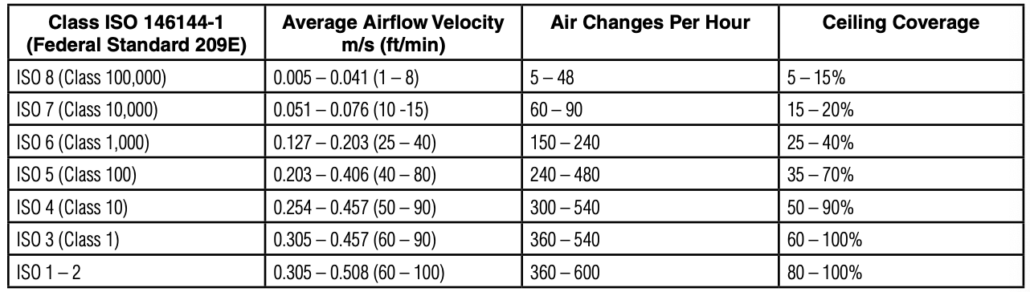
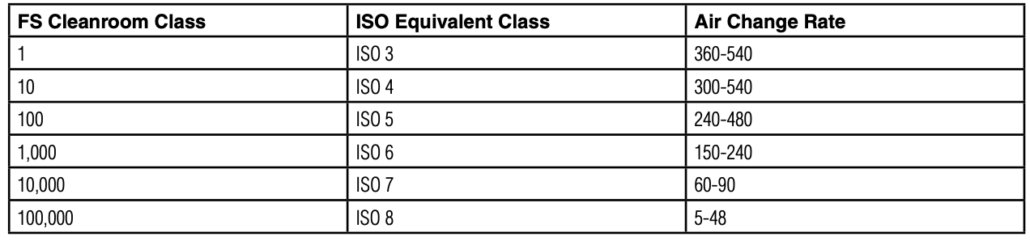
For example, Class 100 under Federal Standard 209E allows 100 particles of 0.5 microns or larger per cubic foot of air, which aligns with ISO Class 5.
Key Factors Influence Cleanroom Classification
A specific cleanroom classification requires strict control of several factors:
1. Air Filtration: Cleanrooms rely on HEPA (High-Efficiency Particulate Air) or ULPA (Ultra-Low Penetration Air) filters to remove particles from the air. HEPA filters can capture 99.97% of particles ≥ 0.3 microns, while ULPA filters capture 99.999% of particles ≥ 0.12 microns.
2. Airflow Patterns and Air Changes: Cleanrooms maintain a continuous filtered air flow , The number of air changes per hour (ACH) is a key cleanliness factor. For example, an ISO Class 5 cleanroom typically has 240–360 air changes per hour.
3. Pressure Control: Cleanrooms use positive pressure to prevent contaminants from entering. For certain applications, such as handling hazardous substances, negative pressure is maintained to keep contaminants contained within the room.
4. Personnel Control and Gowning: Human activity is the largest source of contamination in cleanrooms. Strict gowning protocols (such as wearing sterile gloves, masks, and full-body suits) and movement restrictions are essential to maintain cleanliness.
5. Regular Cleaning: Cleaning schedules must be rigorous. Surfaces, floors, and equipment should be regularly cleaned using specialized tools and techniques to prevent particle buildup.
More Cleanroom Standards to Know
1. ISO 14698: Focuses on biocontamination control, measuring microbial contamination in cleanrooms, and providing guidelines for monitoring and cleaning practices.
2. EU GMP Annex 1: Widely used in pharmaceutical manufacturing, it defines stricter cleanroom standards for sterile products. It complements ISO 14644-1 with specific emphasis on microbial contamination.
3. GMP (Good Manufacturing Practice): These regulations ensure that products are consistently produced and controlled according to quality standards, particularly in industries like pharmaceuticals and food manufacturing.
Choose the Right Cleanroom Classification
Different industries require different levels of cleanliness according to the sensitivity of their products to contaminants:
Pharmaceuticals & Biotechnology: These industries typically use ISO Class 5-7 cleanrooms for manufacturing sterile products and drugs.
Semiconductors: Semiconductor manufacturing requires ultra-clean environments, typically in the range of ISO Class 1-3 to prevent defects caused by microscopic particles.
Medical Devices: Medical device manufacturing often occurs in ISO Class 7-8 environments to ensure product integrity.
Conclusion
Understand and adhere to cleanroom classifications and standards is critical for maintaining contamination control and ensure product quality. Whether you are building a cleanroom for a pharmaceutical plant or managing a semiconductor fab, know the differences between standards like ISO 14644-1 and Federal Standard 209E will help you design, maintain, and monitor a contamination-free environment.
For businesses looking to achieve industry-specific cleanroom standards, working with experts in cleanroom design and air filtration system is crucial for long-term success. Implementing robust filtration systems, proper gowning protocols, and continuous monitoring will help you meet your cleanliness goals and stay compliant with global standards.
If you are involved in manufacturing or research in a sensitive industry, Hope this guide will help you understand what you need to achieve optimal contamination control.